For two ions with charges q1 and q2 separated by distance r, the potential energy canbe calculated from Coulomb's law: E = 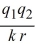 Calculate the energy released when 1 mole NaCl is formed if the constant k = 1.11 × 10-10 C2/J·m, the charge Na+ = +1 e, and the charge on Cl- is -1 e, e = 1.602 × 10-19 C, and
Calculate the energy released when 1 mole NaCl is formed if the constant k = 1.11 × 10-10 C2/J·m, the charge Na+ = +1 e, and the charge on Cl- is -1 e, e = 1.602 × 10-19 C, and 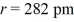
A) +308 kJ/mol
B) +8.20 kJ/mol
C) -297 kJ/mol
D) -494 kJ/mol
E) -371 kJ/mol
Correct Answer:
Verified
Q8: Which of the following solids is likely
Q9: Which ionic solid is likely to have
Q10: Which of the following solids is likely
Q11: Which of the following solids would have
Q12: Which of the following statements is true
Q14: Sodium forms a monatomic ion that has
Q15: Magnesium forms a monatomic ion that has
Q16: Bromine tends to form a monatomic ion
Q17: Sulfur tends to form a monatomic ion
Q18: The octet rule is generally followed for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents