The carbonate ion has the skeletal structure as shown below. Complete the Lewis structure by filling in the bonds and the remaining valence electrons which are not involved in bonds. Which statement made about the carbonate ion is true? 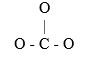 Based on its Lewis structure, the carbonate ion should have ________ resonance hybrids and ________ atoms with formal charges on them in its structure. Hint: Consider the possible places the double bond could go when determining resonance of carbonate.
Based on its Lewis structure, the carbonate ion should have ________ resonance hybrids and ________ atoms with formal charges on them in its structure. Hint: Consider the possible places the double bond could go when determining resonance of carbonate.
A) 2, 2
B) 3, 1
C) 3, 2
D) 2, 1
E) no, 2
Correct Answer:
Verified
Q74: Draw the "best"Lewis structure for sulfur trioxide
Q75: Based on the "best"Lewis structure from formal
Q76: The metaphosphate ion, PO3-, is the structural
Q77: How many resonance structures, if any, can
Q78: The chlorite ion has a Lewis structure
Q80: Draw the Lewis structure of CH3NO2.Based on
Q81: Alcohols belong to a class of organic
Q82: An organic compound has the formula CH3CH2OH.
Q83: An organic compound has the formula CH3CH2NH2.
Q84: Ketones belong to a class of organic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents