A student drew four possible Lewis structures for HBrO4 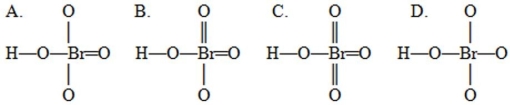 Complete these Lewis structures presented above by filling in the remaining valence electrons that are not in the bonds. Based on these structures, the preferred structure would be the structure shown as ________ in which the sum of the absolute values of the formal charges on all the atoms is ________. Hint: When determining remaining valence electrons remember to account for the electrons already present in the bonds.
Complete these Lewis structures presented above by filling in the remaining valence electrons that are not in the bonds. Based on these structures, the preferred structure would be the structure shown as ________ in which the sum of the absolute values of the formal charges on all the atoms is ________. Hint: When determining remaining valence electrons remember to account for the electrons already present in the bonds.
A) A, 4
B) B, 2
C) C, 0
D) D, 6
E) D, 0
Correct Answer:
Verified
Q153: Use the data to calculate the lattice
Q154: Use the data to calculate the lattice
Q155: Which of the following electron configuration is
Q156: Arrange the following in terms of increasing
Q157: Arrange the following in order of increasing
Q159: The nitrogen monoxide molecule is a unique
Q160: Based on electronegativity considerations, which species should
Q161: Based on electronegativity considerations, which species should
Q162: Based on electronegativity considerations, which species should
Q163: Complete the Lewis structures for COCl2 and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents