Calculate the wavelength, in nanometers, of light emitted by a hydrogen atom when the electron falls from an n = 7 energy level to an n = 4 energy level. Recall that the quantized energies of the levels in the hydrogen atom are given by: 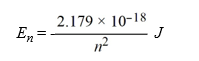
A) 4.45 × 10-20 nm
B) 8.51 × 102 nm
C) 2.17 × 103 nm
D) 1.38 × 1014 nm
E) 2.16 × 103 nm
Correct Answer:
Verified
Q41: Which statement is true concerning Bohr's model
Q42: Which statement about a hydrogen atom is
Q43: Which statement is true of Bohr's equation,
Q44: Calculate the energy required to excite a
Q45: A hydrogen atom starts in the n
Q47: The de Broglie relationship provides a link
Q48: Calculate the wavelength of a particle of
Q49: Calculate the wavelength of an electron (mass
Q50: Calculate the wavelength of a hydrogen atom
Q51: Calculate the wavelength of a helium atom
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents