The three quantum numbers which characterize the solutions to Schrodinger's equation, describing the behavior of the electron in the H atom are usually designated as
A) 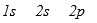
B) 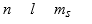
C) 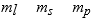
D) 
E) 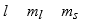
Correct Answer:
Verified
Q49: Calculate the wavelength of an electron (mass
Q50: Calculate the wavelength of a hydrogen atom
Q51: Calculate the wavelength of a helium atom
Q52: The first description of the electron in
Q53: The letter designation for the subshell in
Q55: All orbitals with the same value of
Q56: All orbitals with the same value of
Q57: The notation for the subshell with n
Q58: The notation for the subshell with n
Q59: The notation for the subshell with n
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents