The following are all ionization energies for beryllium. 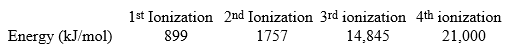 How much energy would be needed to remove three electrons from a ground state beryllium atom?
How much energy would be needed to remove three electrons from a ground state beryllium atom?
Correct Answer:
Verified
Q164: Which atom in the set [Y, Cr,
Q165: Which atom in the set [O, F,
Q166: Which atom in the set [Sr, Fr,
Q167: Which atom in the set [Mg, Cr,
Q168: The following are the ionization energies for
Q170: The frequency of a wave is related
Q171: In an electromagnetic wave, an oscillating charge
Q172: According to the photoelectric effect, increasing the
Q173: A continuous spectrum contains a series of
Q174: Atomic hydrogen has a single electron, and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents