True/False
When n = 2, there are two subshells - the first is for n = 2 and l = 0, while the second is for 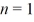 and
and 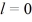
Correct Answer:
Verified
Related Questions
Q176: Bohr's theory was not able to adequately
Q177: The lowest energy state of the hydrogen
Q178: One way in which very small particles
Q179: The amplitude of a wave at any
Q180: For a given value of n, the
Q182: For every value of n, there is
Q183: An electron with an n = 2
Q184: An electron with an n = 3
Q185: The spinning electrical charge of the electron
Q186: The spin of the electron is responsible
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents