Propane is often used to heat homes. The combustion of propane follows the following reaction: 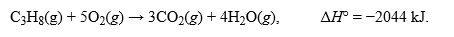 .How many grams of propane must be reacted by this reaction to release 7563 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.
.How many grams of propane must be reacted by this reaction to release 7563 kJ of heat?Hint: Work backwards with moles potentially if you are unsure where to start.
A) 3.70 g
B) 44.1 g
C) 81.6 g
D) 243.4 g
E) 162.8 g
Correct Answer:
Verified
Q55: What would be the "standard state"for hydrogen
Q56: When nitrogen gas reacts with hydrogen
Q57: When aluminum metal reacts with iron(III)oxide
Q58: The thermochemical equation for the reaction between
Q59: The combustion of butane, C4H10, is given
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents
