Complete combustion of hydrocarbons or compounds with only C, H, and O gives CO2 and H2O as the only products. If carried out under standard conditions, the CO2 is a gas and the H2O is a liquid.Given these standard enthalpies of combustion: 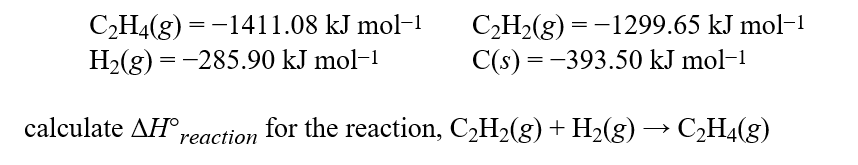 Hint: Remember it is always products minus reactants when performing enthalpy calculations.
Hint: Remember it is always products minus reactants when performing enthalpy calculations.
A) -174.47 kJ
B) +397.33 kJ
C) -961.47 kJ
D) -2424.83 kJ
E) -2996.63 kJ
Correct Answer:
Verified
Q75: Given the equation for the reaction,
Q76: Ammonia gas reacts with molecular oxygen
Q77: The standard enthalpy of combustion for
Q78: A chemical compound has a molecular weight
Q79: The standard heat of combustion for
Q81: Complete combustion of hydrocarbons or compounds
Q82: The energy transferred between objects caused by
Q83: In the equation for the determination of
Q84: In order for a smaller motorcycle to
Q85: What is the mass of an object
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents