Based on the terms and symbols used in the discussions on thermochemistry, the expression, 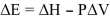 is correct.
is correct.
Correct Answer:
Verified
Q138: Heat energy can always be quantitatively converted
Q139: In an endothermic reaction, the temperature of
Q140: Acid-base neutralization reactions involving strong acids like
Q141: The first law of thermodynamics is
Q142: The first law of thermodynamics is
Q144: For a chemical reaction taking place
Q145: In a bomb calorimeter, no expansion
Q146: The heat of reaction, measured in
Q147: A coffee-cup calorimeter can be used to
Q148: All substances in their "standard state"are at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents