Three metallic elements, copper, magnesium and gold, can be distinguished from one another on the basis of how they react with two strong acids, HNO3(aq)and HCl(aq). Which set below, using the abbreviations R (for reaction occurs)and NR (no reaction)correctly describes what occurs? 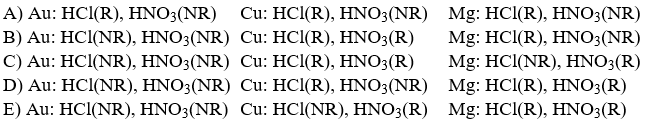
Correct Answer:
Verified
Q65: The activity series of metals is Au
Q66: The activity series of metals is Au
Q67: The activity series of metals is Au
Q68: The activity series of metals is Au
Q69: Three metallic elements, copper, gold and zinc,
Q71: The least reactive metals are those of
A)Group
Q72: The activity series of metals is
Q73: The activity series of metals is
Q74: The activity series of metals is
Q75: The activity series of metals is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents