A solution was made by taking 2.500 g of KMnO4 and dissolving it in enough water to make 1.000 liter of solution. This solution was used to titrate H2C2O4·2H2O, a very pure substance. In acidic media, the reaction is 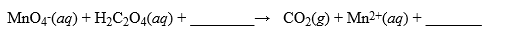 How many mL of this solution are required to titrate a 0.480 g sample of H2C2O4·2H2O? Hint: Remember to use moles as an intermediary when performing your stoichiometry calculations for the titration.
How many mL of this solution are required to titrate a 0.480 g sample of H2C2O4·2H2O? Hint: Remember to use moles as an intermediary when performing your stoichiometry calculations for the titration.
Correct Answer:
Verified
Q165: Rust is a hydrated compound formed between
Q166: Consider the following species: Cl2, Cl-, ClO-,
Q167: Consider the oxidation states of hydrogen in
Q168: In a series of chemical test reactions,
Q169: The activity series of metals is
Q170: Ethanol, C2H5OH, is often found in gasoline.
Q171: Coal is still a large part of
Q173: It is proposed to measure the CO
Q174: A student working on her research project
Q175: A student will prepare a significant quantity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents