In the course of determination of a chemical formula, a student obtained the following mole ratios: 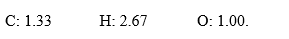 .The empirical formula for the compound is ________.
.The empirical formula for the compound is ________.
Correct Answer:
Verified
Q134: The percent, by weight, of sulfur in
Q135: The percent, by weight, of oxygen in
Q136: How many grams of oxygen are present
Q137: In the course of determination of a
Q138: In the course of determination of a
Q140: In the course of determination of a
Q141: A compound contains 46.46% lithium by mass
Q142: The percent composition by mass of a
Q143: A 1.375 g sample of mannitol, a
Q144: The empirical formula of a compound composed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents