An instructor gives a student a 103.1 g sample of a compound. A student takes this sample and using one of the laboratory balances measures the sample 4 times. He got the following results: 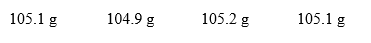 Based on these results, how would the student's measurements best be characterized? Assume that the sample's mass is actually 102.1 g.
Based on these results, how would the student's measurements best be characterized? Assume that the sample's mass is actually 102.1 g.
A) The measurements are both accurate and precise.
B) The measurements are accurate but not precise.
C) The measurements are precise, but not accurate.
D) The measurements are neither accurate nor precise.
E) The measurements say nothing about accuracy or precision.
Correct Answer:
Verified
Q84: When the expression, 412.272 + 0.00031 -
Q85: When the expression, Q86: Evaluate the expression to the correct number Q87: Evaluate the expression to the correct number Q88: 1657.3 grams of a compound are to Q90: An instructor gives a student a 103.1 Q91: A distance of 1.8 × 102 meters Q92: The diameter of a certain atom was Q93: How many micrometers are there in 3.672 Q94: How many mm (millimeters)are there in 6.3![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents