Some students in the AP chemistry class have come up with an idea they would like to have tested, which involves collaboration with several defense facilities. They want to test two small muzzle loading cannons like the ones used in the 18th century. One would be using spherical cannonballs made of iron, while the other would be using spherical cannonballs made of spent uranium. Both cannons will be using cannonballs with a diameter of 5.000 inches. If uranium has a density of 19.05 g/cm3, what would be the mass, in pounds, of the uranium cannonballs? 1 pound = 453.6 g, 1 inch = 2.54 cm.volume = 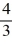 (πr3)Hint: Read the problem carefully. Follow the units and remember that the diameter is twice the radius.
(πr3)Hint: Read the problem carefully. Follow the units and remember that the diameter is twice the radius.
Correct Answer:
Verified
Q214: Solve the following, with the correct number
Q215: Solve the following: (2.15 × 106 +
Q216: If 1 meter = 39.37 inch and
Q217: A home aquarium measures 17.0 inches wide,
Q218: Express 109 miles per hour in meters
Q219: Consider this data from a lab, concerning
Q220: A spherical cannonball made of an iron
Q221: A spherical cannonball made of an iron
Q223: Iron has a density of 7.86 g/cm3.
Q224: Solid antimony has a density of 6.70
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents