Consider the atoms of 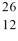 Mg and
Mg and 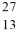 Al. Both of these species have the same
Al. Both of these species have the same
A) number of neutrons and electrons.
B) number of ions.
C) number of neutrons.
D) number of neutrons and mass number.
E) number of protons and electrons.
Correct Answer:
Verified
Q21: Which answer below best describes all atoms
Q22: Which answer below best describes all atoms
Q23: The species shown below that has 24
Q24: The species shown below that has 24
Q25: The species Q27: Consider the atoms of 59Co and 60Co. Q28: An atom of the isotope sulfur-33 consists Q29: An ion of the isotope chlorine-35 has Q30: Compare Q31: A neutral iodine atom has an atomic![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents