The nuclide with the formula 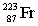 is best described as
is best described as
A) containing 136 neutrons and 87 protons.
B) containing 87 neutrons and having a mass number of 223.
C) containing 223 electrons and 87 protons.
D) containing 87 neutrons and 136 protons.
E) stable.
Correct Answer:
Verified
Q1: The nuclide with the formula 
Q2: Seborgium's most stable isotope contains one-hundred-six protons
Q4: Which force is responsible for repulsions in
Q5: Calculate the binding energy per mole
Q6: Nuclei that lie above the belt
Q7: Nuclei that lie below the belt
Q8: Nuclei that lie below the belt
Q9: Strontium-90 decays by
Q10: 238U is an unstable nuclide that
Q11: 222Rn is an unstable nucleus that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents