Radioactive waste nuclides with short half-lives are usually less of a problem for disposal because they are quite active but only for a short time. For example, 134 Cs has a half-life of 2.1 years. If initially there were 1.5 kg of 134Cs, how much of this nuclide would be present in 14.7 years?(If needed, use the following equation: 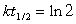
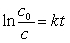 )
)
A) 1.2 kg
B) 120 g
C) 12 g
D) 10 g
E) 14.7 g
Correct Answer:
Verified
Q11: 222Rn is an unstable nucleus that
Q12: Which unstable species decays with the emission
Q13: 64Ni and a positron are formed when
Q14: Fission power produces unstable nuclides which
Q15: Positron emission and electron capture cannot be
Q17: Neutron-capture reactions are
A) endothermic, and only occur
Q18: Which nuclei would undergo neutron capture
Q19: Neutron bombardment of nuclei typically requires lower
Q20: When 60Ni is bombarded with a
Q21: Seaborgium (Z= 106) can be synthesized by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents