Which of the following five complexes are paramagnetic with 2 unpaired electrons? 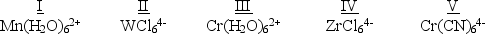 (If needed, use the following equation:Spectrochemical Series
(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) I and II
B) II, IV, and V
C) III
D) IV and V
E) I and III
Correct Answer:
Verified
Q14: You have two samples, one contains [Cr(CN)6]3-
Q15: [Co(NH3)5NO2]Cl2 and [Co(NH3)5ONO]Cl2 are examples of what
Q16: The complexes Co(NH3)63+ and Mo(CO)6 are
Q17: What differences might you expect between the
Q18: Aqueous copper(I) chloride is nearly colourless whereas
Q20: How many chloride ions, Cl-, would you
Q21: Arrange the following complexes in order of
Q22: In which of the following complexes would
Q23: The three main functions of metalloproteins are
A)
Q24: What is the oxidation state of iron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents