Consider the redox process:
Cd(OH)2 + Ni(OH)2 + 2 OH- NiO2 + Cd + H2OWrite the equation for the spontaneous process and determine the free energy change for the spontaneous process. 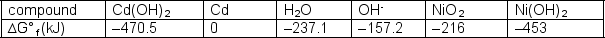
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q46: Use the half-reaction method to balance
Q47: If the coefficient of I- is
Q48: Calculate the standard free energy change
Q49: Draw three molecular pictures illustrating direct electron
Q50: Draw a figure illustrating how a cell
Q52: For the galvanic cell shown in the
Q53: Calculate the standard potential of voltaic cells
Q54: Calculate the standard potential of the
Q55: Calculate the standard potential of the aluminium
Q56: Balance the reaction and calculate the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents