Which of the species listed is the strongest reducing agent? 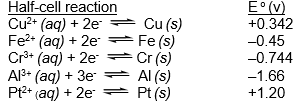 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
Correct Answer:
Verified
Q54: Calculate the standard potential of the
Q55: Calculate the standard potential of the aluminium
Q56: Balance the reaction and calculate the
Q57: An electrochemical cell is constructed that contains
Q58: Which of the species listed is the
Q60: Calculate the standard free energy change for
Q61: Calculate the standard free energy changes
Q62: Calculate the equilibrium constant for the
Q63: Calculate the equilibrium constants for the
Q64: Consider the Daniell cell for which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents