Sodium carbonate, also called soda ash, is often analyzed by titration with strong acid. Initial concentrations of the carbonate ions are about 0.1 M. What indicator(s) would be suitable for detecting the stoichiometric point in this titration? (for H2CO3: pKa1 = 3.75 pKa2 = 10.33) 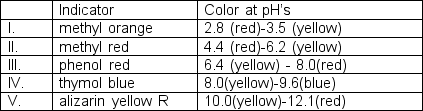
A) I only
B) I and II
C) III only
D) IV only
E) I and V
Correct Answer:
Verified
Q16: In which of the following titrations will
Q17: In which of the following titrations will
Q18: The equivalence point for titration of 50
Q19: The equivalence point for titration of 50
Q20: The equivalence point for titration of 50
Q22: Your friend at Podunk U. urgently emails
Q23: The following graph shows the titration of
Q24: What is the solubility product expression for
Q25: What is the solubility product expression for
Q26: At what pH will an 0.0010 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents