Calculate the pH in the titration of a 0.325 g sample of acetylsalicylic acid (see line drawing below) initially in 25.0 mL water with 0.102 M NaOH when (a) 6.23 mL and (b) 20.00 mL of titrant have been added. 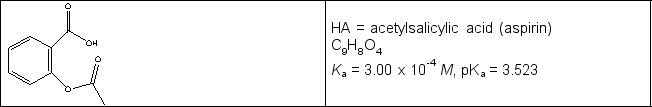
Correct Answer:
Verified
Q38: A buffer solution made from ammonia, NH3,
Q39: What mass of sodium acetate must be
Q40: What mass of ammonium chloride (pKa (NH4+)
Q41: How would you make a buffer of
Q42: How would you make a buffer of
Q44: Calculate the pH in the titration of
Q45: A qualitative sketch of the titration curve
Q46: Consider the titration of 25.0 mL of
Q47: A 5.00 mL sample containing sulphurous acid
Q48: At what pH would a solution with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents