Using the following table, which aqueous 1.0 M solution will have the lowest pH? 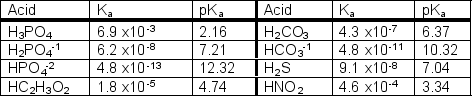
A) Na2CO3
B) NaHS
C) NaC2H3O2
D) NaBr
E) Na3PO4
Correct Answer:
Verified
Q27: Which of the following salt solutions will
Q28: In the reaction between methyl amine, H2N(CH3)
Q29: When 0.10 moles of HCl are added
Q30: Solid NaClO is added to water. What
Q31: What is the pH of a 0.01
Q33: What are the hydronium and hydroxide ion
Q34: If 250 ml of 9.0 M HCl
Q35: What are the hydronium and hydroxide ion
Q36: What are the hydronium and hydroxide ion
Q37: At 99°C, the hydronium concentration of pure
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents