The copper (I) ion is a curious species. In aqueous solutions, there are a number of reactions that it can undergo; one is the reaction with other copper(I) ions:2 Cu+ (aq)  Cu2+(aq) + Cu(s)
Cu2+(aq) + Cu(s) 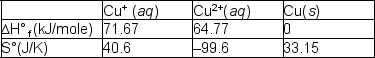 Using the tabulated data, calculate the equilibrium constant for this reaction of Cu+ (aq) at 298 and predict whether it will increase or decrease with increasing temperature. Choose from the following.
Using the tabulated data, calculate the equilibrium constant for this reaction of Cu+ (aq) at 298 and predict whether it will increase or decrease with increasing temperature. Choose from the following.
A) 3.5 x 104; increase
B) 1.2 x 106; decrease
C) 2.2 x 107 increase
D) 2.2 x 107 decrease
E) 3.5 x 104 decrease
Correct Answer:
Verified
Q6: Perform equilibrium calculations on reactions in aqueous
Q7: A system in chemical equilibrium is characterized
Q8: A flask is filled with hydrogen, oxygen
Q9: The equilibrium constant
A) for an aqueous phase
Q10: When is a reaction at equilibrium?
A) when
Q12: Adding water to the reaction vessel
Q13: If the dissolution of CaCl2 is endothermic,
Q14: Which of the following will decrease the
Q15: A system containing nitrogen, ammonia, and hydrogen
Q16: Which way will the Haber process shift
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents