Determine the equilibrium constant at 0oC for the following unbalanced reaction:Ba(OH)2.08H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq) 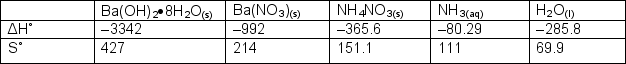
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: Based on the following two reactions
Q42: Calculate the equilibrium constant for the
Q43: The dehydration of benzyl alcohol to benzaldehyde
Q44: Calcite, CaCO3(s), can be converted to CaO(s)
Q45: Calcite, CaCO3(s), can be converted to CaO(s)
Q47: The "water gas shift reaction" is shown
Q48: Consider the dissociation of mono-hydrogen carbonate to
Q49: Water undergoes dissociation as shown below:
2 H2O
Q50: Lead chloride is not very soluble in
Q51: H2PO4-1 is commonly used in making buffer
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents