The Following Reaction Takes Place at 80 Ru(NH3)5(H2O)3+ (Aq) + Cl- (Aq)
the Following Time and Concentration
The following reaction takes place at 80.1°C:
Ru(NH3) 5Cl2+ (aq) + H2O (l) Ru(NH3) 5(H2O) 3+ (aq) + Cl- (aq)
The following time and concentration data are collected: 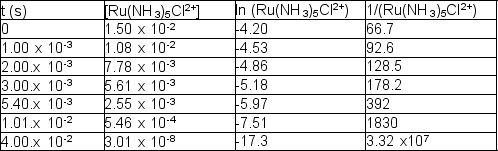 Which of the following is the correct value of the rate constant?
Which of the following is the correct value of the rate constant?
A) 3.28 1/M•s
B) 4.19 1/s
C) 419 1/s
D) 328 1/M•s
E) 328 1/s
Correct Answer:
Verified
Q24: A 1.66 x 10-4 mole sample of
Q25: The reaction A + 2B
Q26: The first-order rate constant for the decomposition
Q27: Butadiene reacts to form its dimmer according
Q28: It is determined that the charcoal in
Q30: Nitrous oxide, N2O, decomposes on metal
Q31: The following are initial rate data for
Q32: The reaction of NO with O2 to
Q33: The following mechanism has been suggested
Q34: What is the rate law associated
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents