The following concentration vs. time data were collected for the reaction:
A + 2B C 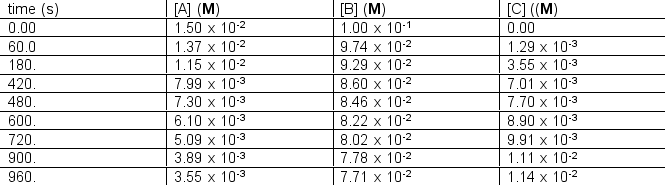 Calculate
Calculate 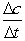 for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
Correct Answer:
Verified
Q40: Write the overall equation of reaction
Q41: Write the overall equation of reaction
Q42: The industrial production of 2-propanol involves the
Q43: Draw a molecular picture showing the termolecular
Q44: Hydrogen peroxide decomposes according to the
Q46: Consider the following three molecular pictures
Q47: The reaction of NO2 with CO to
Q48: What are the units of a rate
Q49: The reaction of NO2 with CO
Q50: The rate law for the reaction of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents