Nitrous oxide, N2O, decomposes on metal surfaces readily at high temperatures following first-order kinetics for the equation:
2 N2O (g) 2 N2 (g) + O2 (g)
The following data are obtained for a reaction at 850°C: 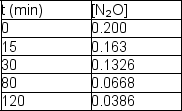 Determine the rate constant and half-life for the reaction.
Determine the rate constant and half-life for the reaction.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q52: Radioactive decay follows first-order kinetics. Some smoke
Q53: Trioxane undergoes decomposition to formaldehyde at
Q54: Ammonium cyanate undergoes rearrangement to form
Q55: Sucrose, cane sugar, reacts with water
Q56: Rate data were collected for the
Q58: The following are initial rate data
Q59: Assume that the following first-order reaction
Q60: The three reactions below, with identical
Q61: Nitrogen dioxide, NO2 will react with
Q62: A proposed mechanism for the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents