The Reaction of Nitrogen Dioxide and Fluorine Is:2 NO2 2 NO2FOne Proposed Mechanism Has Two Steps; the First Step
The reaction of nitrogen dioxide and fluorine is:2 NO2 + F2 2 NO2FOne proposed mechanism has two steps; the first step is rate determining: 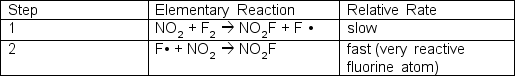 What is the experimentally determined rate law?
What is the experimentally determined rate law?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q58: The following are initial rate data
Q59: Assume that the following first-order reaction
Q60: The three reactions below, with identical
Q61: Nitrogen dioxide, NO2 will react with
Q62: A proposed mechanism for the following
Q64: The rate law for the reaction2
Q65: For the following reaction A +
Q66: In the formation of dinitrogentetroxide, two
Q67: The following rate constants were obtained
Q68: Nitrogen dioxide molecules undergo oxygen exchange with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents