Isomerization of hydrocarbons is important in the refining of petroleum. A simple example is that of butane, which has only two isomers: 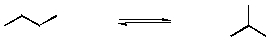
 Which of the following statements is most correct?
Which of the following statements is most correct?
A) At standard temperatures, n-butane is the preferred isomer.
B) At temperatures less than 450, n-butane is the preferred isomer.
C) At temperatures greater than 450ºK, n-butane is the preferred isomer.
D) At ambient temperatures, a sample of n-butane and isobutene which was at equilibrium would contain barely any butane.
E) A 1:1 mixture of butane and isobutane is at equilibrium at room temperature.
Correct Answer:
Verified
Q15: Which of the following selections has the
Q16: The standard molar entropy of NO is
Q17: An important reagent in organic chemistry is
Q18: One process water can undergo is
Q19: A reaction will NEVER be spontaneous
Q21: Consider the following chemical reaction:CaSO4(s) +
Q22: Consider the following unbalanced reaction:CO2(g) + H2O(g)
Q23: Which of the following energy producing technologies
Q24: A child plays with a deck of
Q25: Which of the following processes generate thermal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents