Determine Gibb's Free Energy and if the following unbalanced reaction is spontaneous at 0oC.
Ba(OH)2.8H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq)
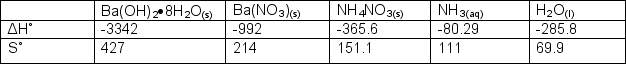
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q49: Calculate the free energy for the
Q50: The conversion of calcite,CaCO3(s), to CaO(s) and
Q51: Determine if the following unbalanced reaction
Q52: What is the reaction quotient for
Q53: Determine Gibb's Free Energy and if
Q55: At what identical concentration of H+
Q56: The possible isomerization for ethanol to methyl
Q57: Complete the following table for determining at
Q58: One of the more important industrial
Q59: Calcite, CaCO3(s), can be converted to CaO(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents