Use the phase diagram of oxygen to determine the phases oxygen passes through starting at 0.001 atm and 100oK, cooling and increasing the pressure steadily to 25 K at 1 atm. 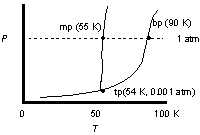
A) gas, liquid
B) liquid, solid
C) gas, liquid, solid
D) liquid, solid, gas
E) gas, solid
Correct Answer:
Verified
Q38: Ruby is a crystalline compound that contains
Q39: Which type of solid is the most
Q40: Below is the structure for zinc sulphide.
Q41: Polonium metal crystallizes in a simple cubic
Q42: Na+ has an ionic radius of 116
Q44: What feature makes the phase diagram of
Q45: The order of increasing melting point for
Q46: A sample of 1.5 moles of CO2
Q47: A tank of nitrogen in a lab
Q48: Some believe that differences between boiling points
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents