Use the following equations for Questions
;
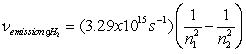 E =
E =
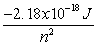 n
n
-A hydrogen atom absorbs a photon resulting in an electron transitioning from the n = 2 to n = 5 energy level. What is the wavelength of the absorbed photon and what is the change in energy of the atom?
A) 434 m, 4.6x10-19 J
B) 434 m, -4.6x10-19 J
C) 434 nm, -4.6x10-19 J
D) 434 nm, 4.6x10-19 J
E) 6.9x1014 s-1, 4.6x10-19 J
Correct Answer:
Verified
Q17: Use the following equations for Questions
Q18: Use the following equations for Questions
;
Q19: Use the following equations for Questions
;
Q20: Use the following equations for Questions
;
Q21: Use the following equations for Questions
;
Q23: Use the following equations for Questions
Ekinetic
Q24: Use the following equations for Questions
Ekinetic
Q25: Use the following equations for Questions
Ekinetic
Q26: Use the following equations for Questions
En
Q27: Use the following equations for Questions
En
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents