Nylon is an important component in many products. One step in the production of nylon is the reaction of cyclohexane with oxygen to give cyclohexanone and water, shown below in the balanced reaction in line formula notation. Determine the energy change for this process using bond energies. (See Table 3-2.) 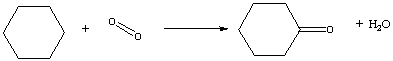
A) ?E = -246 kJ/mol
B) ?E = 246 kJ/mol
C) ?E = -346 kJ/mol
D) ?E = 346 kJ/mol
E) ?E = -305 kJ/mol
Correct Answer:
Verified
Q7: Use the following data for Questions :
Table
Q8: Use the following data for Questions :
Table
Q9: Use the following data for Questions :
Table
Q10: Use the following data for Questions :
Table
Q11: Use the following data for Questions :
Table
Q13: Estimate ?E for the following reaction
Q14: Based on bond energies, determine the reaction
Q15: The reaction of ethene, C2H4, to form
Q16: The combustion of an organic compound
Q17: A constant pressure "coffee-cup" calorimeter is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents