A 1.72 g sample of the saccharide, C6H12O6, shown below is combusted in a constant volume calorimeter of heat capacity 8.21 kJ/K. The initial temperature is 22.1°C. 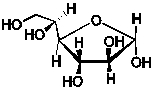 The final temperature is 25.4°C. What is the energy released per mole of this sugar upon combustion? (C = 75.291 J/mol.K for H2O; q = Ccal T)
The final temperature is 25.4°C. What is the energy released per mole of this sugar upon combustion? (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 2830 kJ/mol
B) 3530 kJ/mol
C) 4565 kJ/mol
D) 5865 kJ/mol
E) 4868 kJ/mol
Correct Answer:
Verified
Q19: A constant volume calorimeter's temperature increases
Q20: How much energy is required to
Q21: Lactic acid (line structure below) is
Q22: How much energy in kJ must
Q23: How much energy must be removed
Q25: A piece of cauliflower was combusted
Q26: What is the work (per mole
Q27: The oxidation of sulphur dioxide to
Q28: Determine
Q29: The enthalpy and energy changes for a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents