A 50 g sample of an unknown metal (listed in the below table) is heated to 100°C and placed in 100 g of water at 22.1°C. The final temperature is 29.0°C. Which of the metals listed is the unknown? 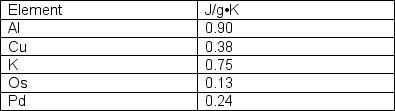
Correct Answer:
Verified
Q55: The solvent dichloromethane, CH2Cl2, is made from
Q56: Using bond energies determine which of CH4,
Q57: Determine the difference in energy between the
Q58: Benzene, C6H6, is a compound whose Lewis
Q59: An experiment in a coffee cup calorimeter
Q60: When 0.891 g of terephthalic acid (line
Q62: A 1200 gram sample of iron is
Q63: Write the chemical equation of the reaction
Q64: A liquid is vaporized at its boiling
Q65: A sample of water is frozen in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents