Consider the van der Waals equation: 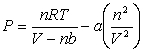
A) the van der Waals "a" term is proportional to molecular size and "b" is proportional to the magnitude of intermolecular forces.
B) the van der Waals "b" term is proportional to molecular size and "a" is proportional to the magnitude of intermolecular forces.
C) large molecules tend to have smaller values for both a and b.
D) the van der Waals equation describes successfully describes the behaviour of non-ideal gases.
Correct Answer:
Verified
Q39: The ideal gas equation is expected to
Q40: Consider the van der Waals "a" coefficient
Q41: Consider the van der Waals "b" coefficient
Q42: If the compressibility factor, pV/nRT, is greater
Q43: If the compressibility factor, pV/nRT is 1
Q45: Athletes are reminded to drink more water
Q46: Ground level ozone
A) forms the ozone layer
Q47: The pressure in an underwater dwelling is
Q48: The level of mercury on the sample
Q49: The level of mercury on the sample
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents