Styrene is used in many plastic products and also in automobile tire rubber. The line structure is shown below: 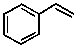 How many hydrogen atoms are contained in 1.05 x 106 kg of styrene?
How many hydrogen atoms are contained in 1.05 x 106 kg of styrene?
A) 4.86 x 1031
B) 6.07 x 1030
C) 1.01 x 107
D) 104
E) 7.71 x 1015
Correct Answer:
Verified
Q24: Cumene is an important industrial chemical used
Q25: Adipic acid, whose line structure is shown
Q26: Calculate the molar mass of terephthalic acid,
Q27: Calculate the molar mass of ammonium phosphate,
Q28: Calculate the molar mass of potassium carbonate,
Q30: One litre of air contains about 1
Q31: How many grams of AgNO3 need to
Q32: Nitric acid is a corrosive acid capable
Q33: Nitric acid, HNO3, is produced along
Q34: Fertilizer is manufactured from, among other
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents