An important reactant in the production of nylon is adiponitrile, the product of addition of HCN and butadiene, the reaction shown below. 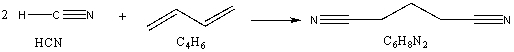 If one obtains 4.50 x 105 kg of adiponitrile from the reaction of 2.50 x 105 kg of butadiene with excess HCN, what is the % yield of the reaction?
If one obtains 4.50 x 105 kg of adiponitrile from the reaction of 2.50 x 105 kg of butadiene with excess HCN, what is the % yield of the reaction?
Correct Answer:
Verified
Q105: Urea is used as a source
Q106: Millions of tons of phosphoric acid
Q107: The following reaction describes the chemistry
Q108: Nitric acid is an important chemical,
Q109: Ethylene glycol is used in automotive antifreeze
Q111: Acetone, most generally known as a component
Q112: The following unbalanced reaction summarizes the
Q113: When 500 ml of 0.75 M
Q114: When 500 ml of 1.75 M
Q115: How many sandwiches can be made from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents