What is the G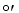 for the reduction of pyruvate to lactate by NADH given the following half reactions?
for the reduction of pyruvate to lactate by NADH given the following half reactions?
Pyruvate- + 2H+ + 2e- lactate- 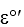 = -0.185
= -0.185
NAD+ + H+ + 2e- NADH 11ee7c6d_c899_8e54_ae82_0910aec0b20b_TBW1044_11= -0.315
A) -96.5 kJ/mol
B) -25.1 kJ/mol
C) -12.5 kJ/mol
D) 25.1 kJ/mol
E) 96.5 kJ/mol
Correct Answer:
Verified
Q1: The oxidation of FADH2 to FAD involves
Q2: Which of the following is the
Q3: If the following half-reactions are combined,
Q4: If the reduction potential for NAD+ is
Q5: In a muscle cell at 37
Q7: What does the reduction potential of 0.815
Q8: What genes are found in the mitochondrial
Q9: Which of the following transport mechanisms is
Q10: Which of the following is a source
Q11: The cytochromes undergo which of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents