With a G 
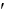 of -16.7 kJ/mol, the reaction catalyzed by hexokinase is considered to be _____.
of -16.7 kJ/mol, the reaction catalyzed by hexokinase is considered to be _____.
A) at equilibrium
B) substrate and product concentration dependent
C) freely reversible
D) metabolically irreversible
E) none of the above
Correct Answer:
Verified
Q1: In eukaryotes, glycolysis typically occurs in the
Q2: The coenzyme _ is the oxidizing agent
Q3: Which of the following represents the net
Q4: In glycolysis, the net gain of ATP
Q6: Phosphoglucose isomerase has a
Q7: In bacterial cells, _ is an activator
Q8: The enzyme responsible for the synthesis of
Q9: Glyceraldehyde-3-phosphate dehydrogenase oxidizes _.
A) an alcohol to
Q10: What residue of phosphoglycerate mutase undergoes covalent
Q11: What is the greatest driving force for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents