What is the intracellular glucose concentration if the G for the following reaction is -20.1 kJ/mol at 37°C and concentrations for glucose-6-phosphate and phosphate are both 1 mM?
Glucose-6-phosphate  glucose + Pi G°
glucose + Pi G° 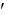 = -13.8 kJ/mol
= -13.8 kJ/mol
A) 1.9 M
B) 87 M
C) 1.9 mM
D) 27 mM
E) 87 mM
Correct Answer:
Verified
Q22: When a reaction is at equilibrium,
Q23: If the
Q24: For the following reaction, calculate the
Q25: For the following reaction, calculate the
Q26: For the following reaction, calculate the
Q28: Which of the following describes the bonding
Q29: If the following reactions were coupled,
Q30: Which of the following factors contributes to
Q31: Which of the following has the most
Q32: In highly active muscle, _ is used
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents