Write the symbolic notation 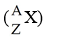 for the following information
for the following information
A)20 protons, 20 electrons, 20 neutrons ________
B)16 protons, 16 electrons, 16 neutrons ________
C)30 protons, 30 electrons, 35 neutrons ________
D)92 protons, 92 electrons, 146 neutrons ________
Correct Answer:
Verified
Q55: After four half-lives the activity of a
Q56: After three half-lives 25 radioactive nuclei remain
Q57: After three half-lives 12.5% of the radioactive
Q58: A nucleotide with a long half-life will
Q59: 1 Ci = 3.7 × 1010 Bq
Q61: Complete the following table: Q62: Write the symbol for the isotope of Q63: The element rubidium (Rb)comprises two naturally occurring Q64: The element magnesium (Mg)comprises three isotopes with Q65: Complete the following table.
![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents