Complete the following equations with the symbol for the atom or particle represented by the blank space. Show the mass numbers and atomic numbers of the isotopes formed or the symbols of the subatomic particles:
A)  B)
B) 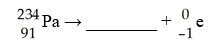 C)
C) 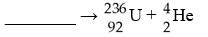 D)
D) 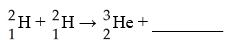 E)
E) 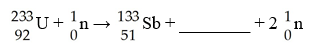 F)
F) 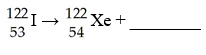 G)
G) 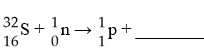
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q61: Complete the following table: Q62: Write the symbol for the isotope of Q63: The element rubidium (Rb)comprises two naturally occurring Q64: The element magnesium (Mg)comprises three isotopes with
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents