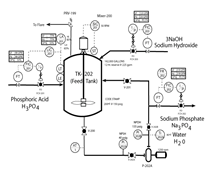
Given the chemical equation: H3PO4 + 3 NaOH→Na3PO4 + 3H2O
If you are told to increase H3PO4 from 98 GPM to 294 GPM, what will the settings be for the other reactant and products? Show your work
. 
Correct Answer:
Verified
Q57: 2H3PO4 --» H2O + H4P2O7. Is
Q58: Uses a porous solid to remove gases
Q59: _ removes one or more components from
Q60: The _ can be found in the
Q61: A 2500 pound barrel has a 37%
Q63: A(n) _ is the smallest particle of
Q64: An acid is:
A) a bitter tasting chemical
Q65: 2Na2O + 2HOCl --» 2NaOCl + H2O.
Q66: The major difference between the two most
Q67: The chemical name for Gold:
A) Ag
B) Au
C)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents