Solve the problem.
-Let y(t) be the concentration of a substance in a chemical reaction. The change in the concentration, under appropriate conditions, is modeled by the equation 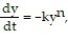 where k > 0 is a rate constant and the positive integer n is the order of the reaction. Solve the initial value problem for a third-order reaction
where k > 0 is a rate constant and the positive integer n is the order of the reaction. Solve the initial value problem for a third-order reaction 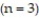 , assuming y(0) = 4 and k = 0.6.
, assuming y(0) = 4 and k = 0.6.
A) y = 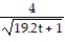
B) y = - 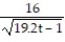
C) y = 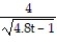
D) y = 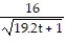
Correct Answer:
Verified
Q43: Solve the initial value problem and leave
Q44: Solve the initial value problem and leave
Q45: Solve the initial value problem and leave
Q46: Solve the initial value problem and
Q47: Solve the problem.
-The spread of a cold
Q49: Find the general solution of the equation.
Q50: Find the general solution of the equation.
Q51: Find the general solution of the equation.
Q52: Find the general solution of the equation.
Q53: Solve the initial value problem.
-y'(t) + 9y
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents