Multiple Choice
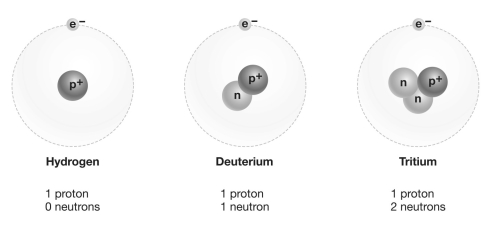 Refer to the figure above and then answer the question that follows.
Refer to the figure above and then answer the question that follows.
-Consider carbon-12, carbon-13, and carbon-14 (the numbers indicate atomic mass) . Which of these forms of carbon is an isotope?
A) carbon-12
B) carbon-13
C) carbon-14
D) None are isotopes.
E) All are isotopes.
Correct Answer:
Verified
Related Questions
Q1: The subatomic particles with the most mass
Q2: If the atomic number is 18, then:
A)
Q3: The mass of matter is proportional to
Q4: Which of the following is not a
Q5: Isotopes have been used to:
A) determine the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents