refer to the reaction
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.
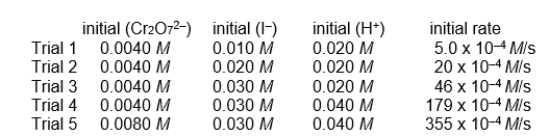
-The rate law for this reaction would be:
A) zero-order in Cr2O72-
B) half-order in Cr2O72-
C) first-order in Cr2O72-
D) second-order in Cr2O72-
E) none of the above
Correct Answer:
Verified
Q32: refer to the reaction for which
Q33: refer to the reaction for which
Q34: refer to the reaction for which
Q35: refer to the reaction for which
Q36: refer to the reaction
Cr2O72-(aq) + 6
Q38: refer to the reaction
Cr2O72-(aq) + 6
Q39: refer to the reaction
Cr2O72-(aq) + 6
Q40: refer to the reaction
Cr2O72-(aq) + 6
Q41: A certain substance, initially at 0.10 M
Q42: The reaction A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents