Consider the following reaction:
H2PO4-(aq) + HCO3-(aq) 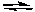 H2CO3(aq) + HPO42-(aq)
H2CO3(aq) + HPO42-(aq)
Brønsted would identify the acidic species as:
A) H2PO4- and H2CO3
B) H2PO4- and HPO42-
C) HCO3- and H2CO3
D) HCO3- and HPO42-
E) H2CO3 and HPO42-
Correct Answer:
Verified
Q1: Which of the following compounds is not
Q2: Which of the following compounds is not
Q4: Which compound cannot act as a Brønsted
Q5: Which of the following compounds cannot be
Q6: Which of the following isn't a Brønsted
Q7: What is the conjugate acid of hydrogen
Q8: What is the conjugate base of thiocyanic
Q9: The dihydrogen phosphate ion, H2PO4 - ,
Q10: Ethanol (CH3CH2OH), like water, can behave both
Q11: What is the conjugate base of HSO4-?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents